Jackie Kraft, RN, Value Analysis Manager & Clinical Resource Manager, Huntsville Health
Gina Holcombe RN, BSN, Director of Surgery Main, Third & GMT, Huntsville Health
Halit O. Yapici, MD, MBA, MPH, Senior Consultant, Boston Strategic Partners
Importance of Hemostats and Cost Control
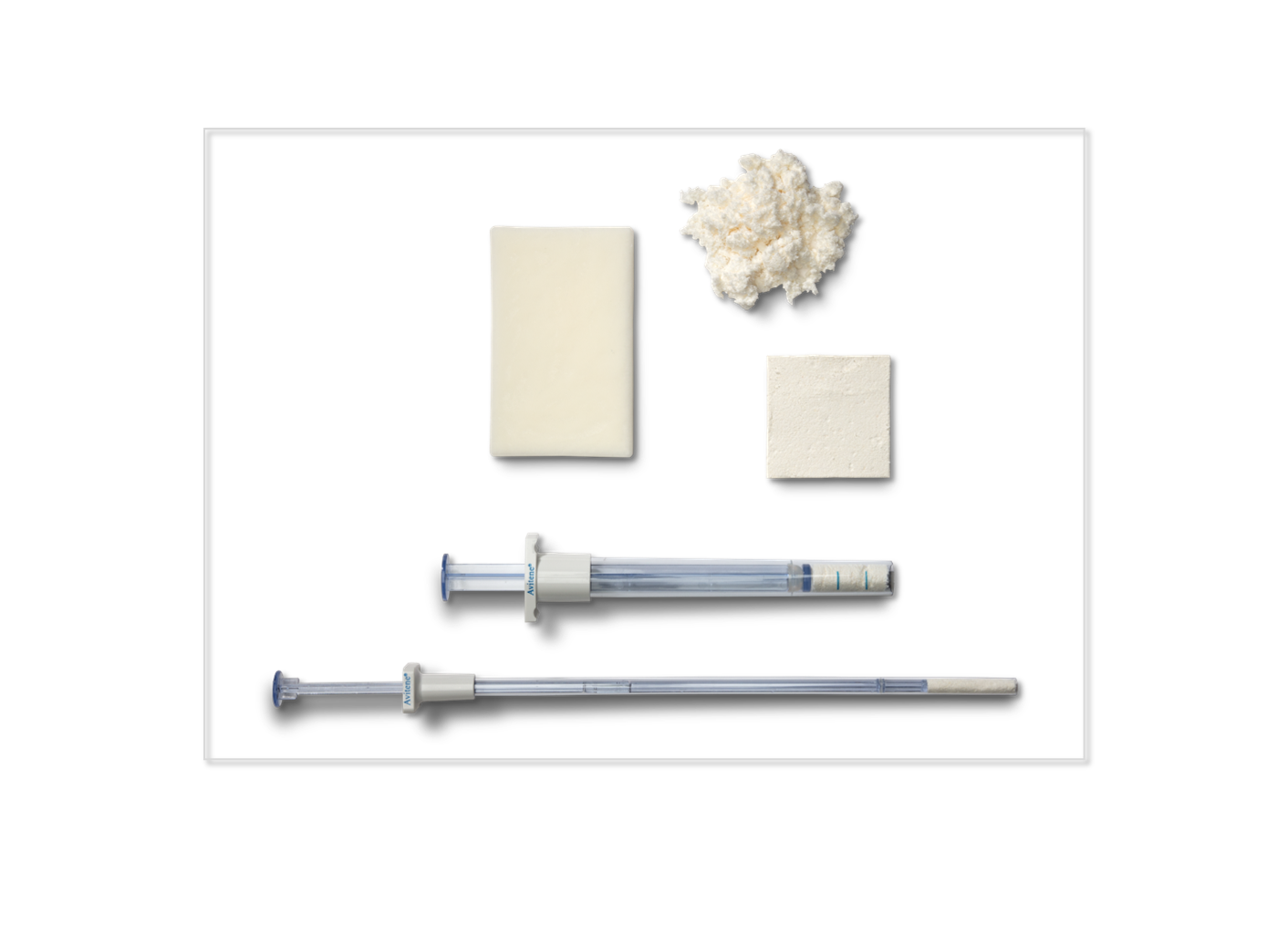
Bleeding-related complications occur in around one third of all surgery cases which may lead to higher rates of transfusion, longer length-of-stay, and higher treatment costs.1 Historically, intraoperative bleeding was controlled through suturing, electrocautery, and surgical clips. These earlier techniques were later supplemented by topical hemostats9 which are categorized into four main groups based upon their primary components: (I) mechanical hemostats that form a matrix on the bleeding site and activate the extrinsic coagulation cascade; (II) active hemostats that rely on thrombin to control bleeding; (III) flowable hemostats that contain a gelatin matrix and are dispensed as a flowable structure directly into a wound; and (IV) fibrinogen/fibrin hemostats work by creating a sealing barrier that prevents the leakage of gas or liquid from a structure.2 One study evaluating 3.6 million surgical cases found that topical hemostats were used in 30% of all surgeries, ranging between 82.2% for spinal surgery to 6.6% for prostatectomy cases. The use of topical hemostats increased by 24% between 2000 and 20103 and the market for hemostats is expected to continue growing at a compound annual growth rate of 6.0% into 2023.4
Creating an optimal supply chain requires close collaboration between clinicians, hospital management, suppliers, and distributors. Supply chain management is particularly challenging in surgical environments, which accounts for 50% of total hospital inventory and 60% of total hospital costs.5 Furthermore, operating rooms have a high number of physician preference items (PPI), which are supplies/devices for which physicians exhibit personal preference.6 Although the hospital is the actual purchaser of the PPI inventory, physicians determine the device for a particular patient and procedure. PPIs often lead to conflict since surgeons’ preferences may be based on factors such as familiarity and convenience while hospitals may prioritize cost containment.7
Hospitals are particularly sensitive to the costs regarding PPI inventory due to budgetary pressures from shrinking reimbursements (i.e., bundled payments) and increased use/cost of medical devices.6 Volume-based contracts may be a viable strategy to control costs; however, contract compliance can be particularly challenging for the PPIs due to each surgeon’s clear and varied preferences.7 Thus, any initiative that includes PPIs requires utmost collaboration and communication among clinical and non-clinical stakeholders.
Huntsville Health: A Hospital System’s Experience
Huntsville Hospital Health System is a public, not-for-profit hospital system in Alabama. Centered on the 971-bed main campus in downtown Huntsville, the health system includes more than 2,200 patient beds and 15,000 employees. Huntsville Health is the second largest hospital system in Alabama and is one of three Level I Trauma Centers in the state. In addition to a central campus consisting of a main hospital, Women and Children’s hospital, and various outpatient medical facilities, Huntsville Health is also affiliated with eight other hospitals in neighboring counties.
As a large hospital system, it has been essential for Huntsville Health to manage inventory and control costs in order to maintain financial sustainability. With 62 operating rooms and over 150 surgical cases per day at the main campus alone, Huntsville Health has been actively pursuing opportunities to control costs while improving patient outcomes in the surgical setting.
The Initiative at a Glance
Before 2018, Huntsville Health bought hemostatic products from a previous vendor for decades. Hemostats are characterized by their low per-unit cost and high-volume use. Therefore, the aggregate financial impact of these items is often overlooked. However, low-cost high-volume items may present a significant savings opportunity in a large hospital system such as Huntsville Health which performs thousands of surgical procedures per year.
Starting in 2018, Huntsville Health gradually switched to lower-cost and clinically comparable hemostat products across all facilities in the health system which led to significant cost savings. The successful planning, evaluation, and implementation of the initiative relied on coordination and communication among clinical and non-clinical stakeholders at Huntsville Health, as well as support of the BD representatives. The project team planned for multiple opportunities to receive detailed feedback from clinical stakeholders, which led to significant adjustments throughout the initiative to ensure a smooth transition without harming physician satisfaction and patient experience. Huntsville Health achieved significant cost savings and improved efficiency by switching to lower-cost alternatives, standardizing the hemostat portfolio, and eliminating the need for thrombin.
Championing an Initiative
In 2018, Huntsville Health was presented with a significant cost savings opportunity to acquire lower-cost and clinically comparable hemostat products from BD through a volume-based contract. This opportunity presented two unique challenges that set the initiative apart from previous initiatives at Huntsville Health. First, a top-down approach to changing items involving physician preferences was likely to cause significant pushback from the surgeons. Second, a system-wide adoption was absolutely necessary to receive volume-based rebates and realize significant cost savings. Past experiences at Huntsville Health were bottom-up initiatives where physicians demanded a change in a single surgical department or facility. When the adoption was system-wide, it was mainly commodity items (e.g., surgical gloves) which did not involve products with significant clinician preferences.
The project champions recognized that a system-wide adoption of products involving physician preferences would require considerable benefits to offset the expected challenges in implementation. Therefore, they performed a cost-analysis to estimate the potential cost savings which played a key role in gaining support from the stakeholders, including the hospital leadership. To perform the cost-analysis, the team collected hemostat utilization data based on an existing electronic preference card system tracking the type/volume of hemostats used by each surgeon. The analysis results estimated significant cost savings which gave the team the green light to move forward with planning the system-wide implementation.
Planning for Challenges
The project team made several key decisions to address the unique challenges previously identified.
First, the team placed a strategic emphasis on receiving and analyzing feedback from as many clinicians as possible. Although the project team had detailed information regarding hemostat usage by each surgeon, they lacked qualitative information regarding physician preferences. Therefore, the team planned for multiple phases (i.e., product showcasing, product evaluation) to receive feedback from the surgeons and fine-tune the initiative.
Second, the team had to plan for the scale of a system-wide implementation. A simultaneous implementation at all facilities in the health system would stretch the resources thin. Thus, the team decided on an iterative approach, moving from facility to facility within each phase of the initiative. This approach allowed the project team to be fully present in each facility for a sufficient amount of time throughout the three main phases: showcasing, evaluation, and implementation.
Phase I – Showcasing the Product Portfolio
During the first phase, the hemostat products from the prospective portfolio were showcased to clinical stakeholders in each facility for over a month-long period. Rooms/hallways in facilities were used to exhibit the products where surgeons could walk in to observe and ask questions. Furthermore, the project team hosted meetings for entire surgical teams at several facilities to provide information about the prospective product portfolio.
There were two main objectives of the showcasing phase. The first objective was to give physicians an opportunity to familiarize themselves with the new products. The second objective was to collect in-depth feedback regarding surgeon preferences about the prospective portfolio. Clinicians asked questions, voiced their concerns, and provided crucial information which was reviewed by the project team on a weekly basis.
Overall, several stakeholders emphasized the importance of the product showcasing phase, which provided crucial information for successful implementation of the initiative.8 For example, the initial plans did not include flowable hemostat products. After discovering the importance of these products for multiple surgeons, Huntsville Health immediately addressed the gap in the prospective product portfolio by acquiring flowable hemostats in a cost-effective manner from a third vendor. The team also identified specific sizes of hemostat products that were not utilized by surgeons which allowed SKU reduction to increase efficiency.
Phase II – Evaluation
The team moved to the evaluation phase after adjusting the prospective product portfolio based on their findings in the showcasing phase. Phase II began at the largest Huntsville Health facilities to evaluate the feasibility of a system-wide adoption. Potential major issues at large facilities (i.e., the main campus) would allow for the team to reconsider the plans for implementation. The prospective hemostat products were evaluated on a surgeon-by-surgeon basis at each facility where the project team was present in the surgical units to provide information, collect feedback, and identify potential issues. Overall, the evaluation phase lasted for two months which was longer than the team initially anticipated; however, it was crucial to give surgeons enough time to try the products and provide detailed feedback.
Clear communication between the system-level project champions and local stakeholders was essential to collecting and addressing feedback throughout the initiative. Most surgeons were receptive to evaluating and providing feedback regarding the prospective hemostat product portfolio. The project team reiterated the clinical/economic benefits of the initiative to surgeons who initially were not interested in trying the new products. After the evaluation, the surgeons who had a strong preference for the previous products were carved out from the implementation which permitted these physicians to continue using hemostats from the previous vendor while allowing Huntsville Health to remain compliant with contractual obligations.
Phase III – Implementation
After a successful evaluation phase demonstrating overall support for the prospective hemostat portfolio among stakeholders, Huntsville Health moved forward to the system-wide implementation phase. Unlike the evaluation, the implementation started at the smaller facilities which allowed faster implementation (due to lower inventory volume) to realize cost savings sooner. Each facility transitioned to ordering and stocking BD hemostat products while maintaining a small stock for the previous products. Smaller facilities completed the product transition fairly quickly, while large facilities, including the main campus, are improving their compliance gradually.
Sustainability and Outcomes
Since the implementation, Huntsville Health has achieved over 80% compliance and significant direct cost savings. The health system has also observed indirect cost savings from improved efficiency due to product standardization/SKU reduction. Unique advantages of the new product portfolio have led to additional improvements in efficiency by eliminating the need for stocking/using thrombin and decreasing the time needed for pre-surgery preparation. The cost savings are expected to increase as facilities are gradually increasing their compliance rates. The stakeholders have not observed any changes in patient outcomes despite decreased costs. Please note that a successful conversion requires persistence and consistent follow-up from the BD sales representative to ensure all parties are following the new policy. Coordination with the Materials Manager is important, and routine check-in to ensure competitor SKUs are not being used.
Overall, stakeholders at Huntsville Health are satisfied with the initiative and would recommend similar initiatives to other institutions with a high-volume of surgery load. The project team is currently focused on sustaining and improving the benefits of the initiative. The team is actively collecting post-implementation feedback from the clinical stakeholders which are discussed at bi-monthly meetings to identify/address any new challenges. BD’s involvement has also been crucial to successful implementation and sustainability of the initiative.
Vendor Involvement
BD’s collaboration was instrumental in the implementation and sustainability of this initiative. From the very beginning, BD representatives were a vital strategic resource for the project team. According to key project champions at Huntsville Health, BD representatives were knowledgeable, available, and enthusiastic team members in the changeover process.
BD’s support consisted of:
- Providing in-depth information on BD hemostats which helped the team identify clinical equivalencies between previous and prospective hemostat portfolios
- Performing product education/training for surgeons and other clinical staff throughout the initiative and answering any specific questions about the products
- Ongoing bimonthly meetings with Huntsville Health team to monitor compliance and provide continuing education for clinical users
Key Factors for Success
Two interrelated challenges emerged throughout the initiative. First, hemostat products are PPIs and therefore, the transition required interactions with numerous surgeons for successful implementation. Second, the success of the changeover was dependent upon system-wide compliance due to volume-based contracting and the low-cost high-volume nature of hemostats. The challenges related to the surgeon preferences were magnified by the scale and volume of the changeover. In fact, this hemostat changeover was the largest scale initiative Huntsville Health has ever implemented.8
1. Communication and Feedback
The team tackled these challenges by planning for multiple opportunities to receive feedback from stakeholders throughout the initiative. The feedback from showcasing and evaluation phases allowed adjustments to the prospective portfolio to ensure smooth transition and overall success. Weekly meetings with facility-level directors and OR managers helped the project team in addressing roadblocks as they arose. These efforts paid off as many potential challenges (i.e., gaps in prospective portfolio, individual surgeons with strong preferences) were identified and addressed by fine-tuning the implementation plans.
2. Strategic Planning
Strategic planning was another key to success due to complexities in logistics. Implementing a product changeover in a large health system with facilities of varying sizes and locations (i.e., rural and urban) was not an easy task. Furthermore, many surgeons performed procedures at multiple facilities within the health system during specific days or weeks. The team had developed a clear roadmap allowing each facility to have sufficient time to showcase, evaluate, and implement the initiative while giving every surgeon an opportunity to provide feedback.
3. Vendor Involvement
Finally, the collaboration and involvement of the vendor was essential to the success of the initiative. The literature highlights the importance of vendor involvement in product changeover initiatives involving PPIs since surgeons’ communication with vendor representatives is often key to forming their preferences and influencing their choices of products.7 In this case, the information provided by BD representatives regarding their products allowed surgeons to make educated decisions to ensure the high-quality and cost-effective care for their patients.
Overall, this initiative demonstrated that a top-down system-wide PPI changeover initiative can bring success and achieve significant cost savings. Through collaboration and communication among clinical and non-clinical stakeholders, Huntsville Health was able to reach over 80% compliance in less than two years. With its unique intersection of challenges, this example illustrates effective strategies to adopt a PPI portfolio including clear communication, strategic planning, and vendor involvement. Health systems with a high number of surgeries may consider lower-cost alternatives for high-volume surgical products with relatively low per-unit costs.
Citations
- Stokes M, Ye X, Shah M, et al. (2011). Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res, 11 (135).
- Schreiber, M., & Neveleff, D. (2011). Achieving Hemostasis With Topical Hemostats: Making Clinically and Economically Appropriate Decisions in the Surgical and Trauma Settings. AORN Journal, 94(5), S1-S20.
- Wright, J., Ananth, C., Lewin, S., Burke, W., Siddiq, Z., Neugut, A., . . . Hershman, D. (2014). Patterns of use of hemostatic agents in patients undergoing major surgery. Journal of Surgical Research, 186(1), 458-466.
- Markets and Markets. (2018). Market Research Report: Hemostats Market Global Forecast to 2023.
- Ahmadi, E., Masel, D., Metcalf, A., & Schuller, K. (2019). Inventory management of surgical supplies and sterile instruments in hospitals: A literature review. Health Systems, 8(2), 134-151.
- Burns, L., Housman, M., Booth, R., & Koenig, A. (2018). Physician preference items: What factors matter to surgeons? Does the vendor matter? Medical Devices (Auckland, N.Z.), 11, 39-49.
- Montgomery, K., & Schneller, E. (2007). Hospitals’ Strategies for Orchestrating Selection of Physician Preference Items. Milbank Quarterly, 85(2), 307-335.
- Interviews with two Clinical and Managerial Leaders at Huntsville Health and a surgical specialist at BD. In: Yapici HO, ed2019.
- Chiara, O., Cimbanassi, S., Bellanova, G., et al. (2018). A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg, 18(68).